66. Delar, E.; Cloutier, M.;
Gauthier, C.* Exploring anthocyanins
and their derivatives: synthesis, physicochemical properties, and
therapeutic potential. In:
Studies in Natural Products Chemistry (Bioactive Natural Products), 1st edition, Ed.: Atta-ur-Rahman, Elsevier,
2025, accepted (invited contribution)
65.
Verma, N.; Cloutier, M.; Clément, D.; Gauthier, C.* Synthesis of α-
D-idose pentaacetate from
β-D-glucose pentaacetate via Paulsen acetoxonium rearrangement. In:
Carbohydrate Chemistry: Proven Synthetic Methods, Volume 6, Ed.: Giguère, D.; Vincent, S. P.;
2025, CRC Press, accepted (invited contribution)
64. Gamboa Marin, O. J.; Verma, N.; Cloutier, M.;
Gauthier, C.* Synthesis of rhamnose-modified Lewis-X-containing saponins.
Eur. J. Org. Chem. 2025, e202500285 (invited contribution) (
link) (
SI)
63. Gamboa Marin, O. J.; Ng, K.; Verma, N.; Yapi, A. G. F.; Pantophlet,
R.;
Gauthier, C.* Lewis-X-containing triterpenoid saponins inhibit
DC-SIGN- and L-SIGN-mediated transfer of HIV-1 infection.
Chem. Eur. J. 2025, e202500993 (
link) (
SI)
62.
Gamboa Marin, O. J.; Adda-Bouchard, Y.; Sylla, B.; Verma, N.;
Charpentier, T.; Huber, M.; Lopez, G.; Pichette, A.; Lamarre, A.;
Gauthier, C.* Immunological
and toxicological assessment of triterpenoid saponins bearing Lewis-X- and QS-21-based trisaccharides.
Chem. Eur. J. 2025, e202500994 (
link) (
SI)
61. Gupta, S.;
Gauthier, C.* 1-Thiosugars: from synthesis to applications.
Curr. Org. Chem. 2025,
29, 359 (invited contribution to the special issue "Chemistry and biology of carbohydrates") (
link)

60. Delar, E.; Tigherghar, Y.; Girard, L.; Haddad, M.; Ramassamy, C.;
Legault, J.;
Gauthier, C.* Synthesis and pharmacological evaluation of
nature-inspired phenacyl glycosides.
Carbohydr. Res. 2024,
545, 109281 (invited contribution to the special issue in honor of Prof. Richard R. Schmidt's 90th anniversary) (
link) (
SI)

59. Cao, J.; Veytia-Bucheli, J. I.; Liang, L.; Wouters, J.;
Silva-Rosero, I.; Bussmann, J.;
Gauthier, C.; De Bolle, X.; Groleau,
M.-C.; Déziel, E.; Vincent, S. P. Exploring fluorinated heptose
phosphate analogues as inhibitors of HldA and HldE, key enzymes in the
biosynthesis of lipopolysaccharide.
Bioorg. Chem. 2024,
153, 107767 (
link) (
SI)
58. Sylla, B.; Jost, G.; Lavoie, S.; Legault, J.;
Gauthier, C.*; Pichette, A. Synthesis and cytotoxicity evaluation of
D- and
L-sugar-containing mono- and bidesmosidic ursane-type saponins.
Bioorg. Med. Chem. 2024,
106, 117737 (
link) (
SI)
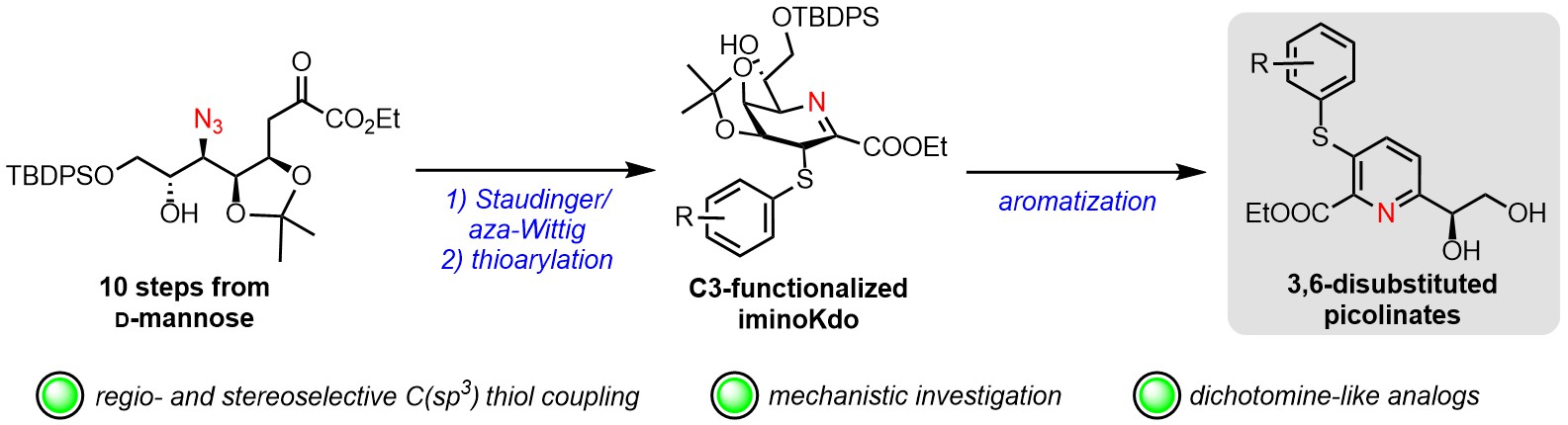
57. Manmode, S.; Hussain, N.; Gamboa Marin, O. J.; Kato, A.;
Veytia-Bucheli, J. I.; Vincent, S. P.;
Gauthier, C.* Thioarylation of
6-amino-2,3,6-trideoxy-
D-
manno-oct-2-ulosonic acid (iminoKdo): access to 3,6-disubstituted picolinates and mechanistic insights.
Chem. Eur. J. 2024,
30, e202303904 (invited contribution)
(
link) (
SI)
56.
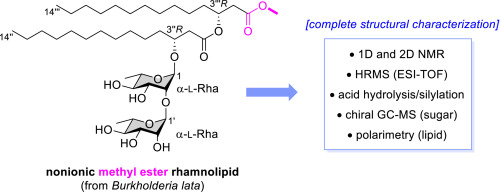
Gauthier, C.*; Lavoie, S.; Kubicki, S.; Piochon, M.; Cloutier, M.; Dagenais Roy, M.;
Groleau, M.-C.; Pichette, A.; Thies, S.; Déziel, E.
Structural characterization of a nonionic rhamnolipid from
Burkholderia lata.
Carbohydr. Res. 2024,
535, 108991 (
link)
55. Deimel, L. P.; Xue, X.; Khan, A.; Moynie, L.; Buchanan, C. J.; Sun, G.; McBride, R.; Schuster, H.;
Gauthier, C.;
Saliba, R.; Leonavicus, K.; Minall, L.; Bort, G.; Russell, R. A.;
Sezgin, E.; Paulson, J. C.; Anthony, D. C.; Baldwin, A. J.; Naismith,
J.; Schiffner, T.; Davis, B. G.; Sattentau, Q. J. Engineered display of
ganglioside-sugars on protein elicits a clonally and structurally
constrained B cell response.
bioRxiv 2023 (
link)
54. Verma, N.; Cloutier, M.;
Gauthier, C.* Thioglycoside-based glycosylations in oligosaccharide synthesis. In:
Synthetic Strategies in Carbohydrate Chemistry, 1st edition, Ed.: Tiwari, V. K.;
2023, Elsevier, 747 pp (invited contribution) (
link)
53.
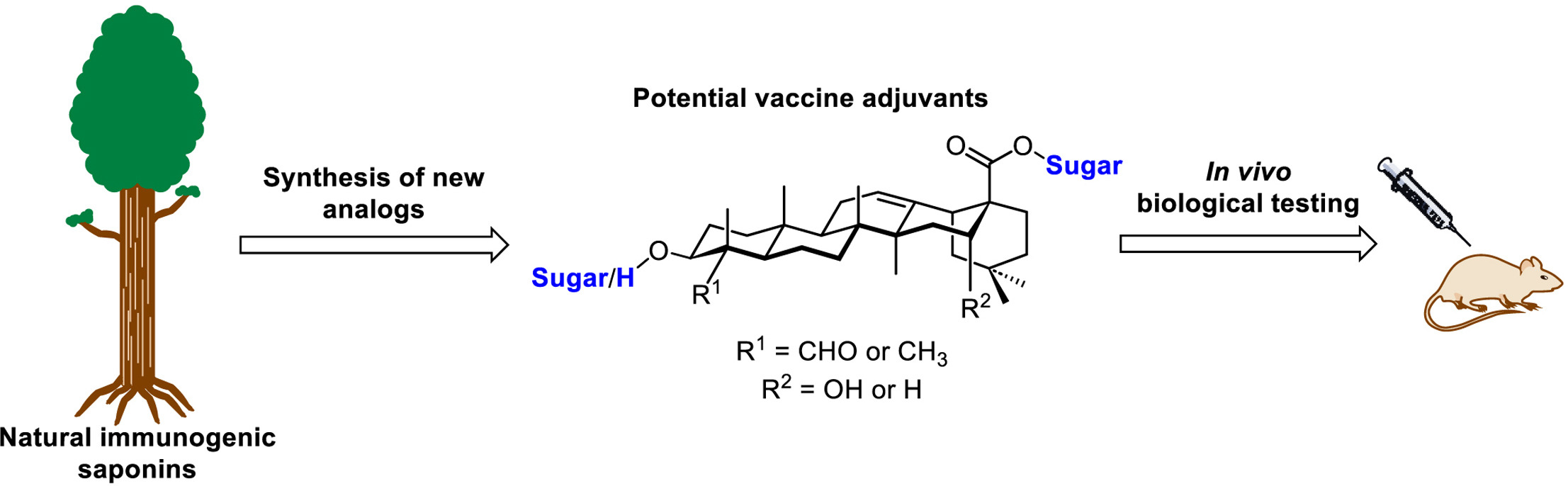
Gamboa Marin, O. J.; Heis, F.;
Gauthier, C.* Synthesis of
immunostimulatory saponins: a sweet challenge for carbohydrate
chemists.
Carbohydr. Res. 2023,
530, 108851 (invited contribution) (
link)
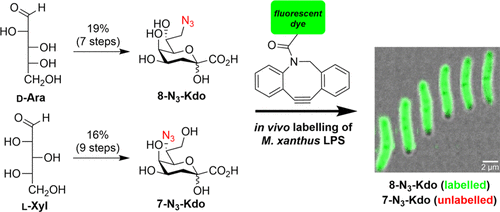
52. Saïdi, F.; Gamboa Marin, O. J.; Veytia-Bucheli, J. I.; Vinogradov,
E.; Ravicoularamin, G.; Jolivet, N. Y.; Kezzo, A. A.; Ramirez Esquivel, E.; Panda, A.; Sharma, G.; Vincent, S. P.;
Gauthier, C.*; Islam, S. T. Evaluation of azido
3-deoxy-
D-
manno-oct-2-ulosonic acid (Kdo) analogues for click chemistry-mediated metabolic labelling of
Myxococcus xanthus DZ2 lipopolysaccharide.
ACS Omega 2022,
7, 34997-35013 (
link) (
SI)
51. Cloutier, M.; Lavoie, S.;
Gauthier, C.* C7 epimerization of benzylidene-protected
β-D-idopyranosides brings structural insights into idose conformational flexibility. J. Org. Chem. 2022, 87, 12932-12953 (link) (SI)
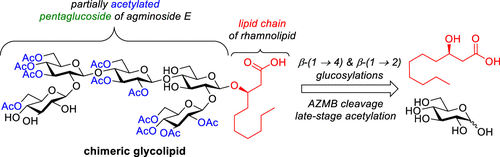
50. Muru, K.; Cloutier, M.; Provost-Savard, A.; Di Cintio, S.;
Burton, O.; Cordeil, J.; Groleau, M.-C.; Legault, J.; Déziel, E.;
Gauthier, C.* Total
synthesis of a chimeric glycolipid bearing the partially
acetylated backbone of sponge-derived agminoside E.
J. Org. Chem. 2021,
21, 15357-15375 (
link) (
SI)
49.
Schellenberger,
R.; Crouzet, J.; Nickzad, A.; Shu, L.-J.; Kutschera, A.; Gerster, T.; Borie, N.;
Dawid, C.; Cloutier, M.; Villaume, S.; Dhondt-Cordelier, S.; Hubert,
J.; Cordelier, S.; Mazeyrat-Gourbeyre, F.; Schmid, C.; Ongena, M.;
Renault, J. H.; Haudrechy, A.; Hofmann, T.; Baillieul, F.; Clément, C.;
Zipfel, C.;
Gauthier, C.; Déziel, E.; Ranf, S.; Dorey, S. Bacterial rhamnolipids
and their 3-hydroxyalkanoate precursors activate
Arabidopsis innate immunity through two independent mechanisms.
Proc. Natl. Acad. Sci. USA 2021, 118, e210136118 (
link)
48.
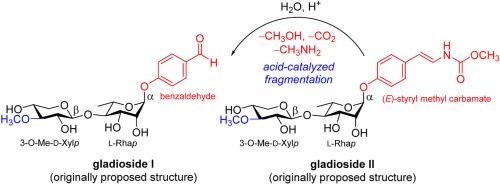
Hussain, N.;
Delar, E.; Piochon, M.; Groleau, M.-C.; Tebbji, F.; Sellam, A.; Déziel,
E.;
Gauthier, C.* Total synthesis of the proposed structures of
gladiosides I and II.
Carbohydr. Res. 2021,
507, 108373 (invited contribution to the special issue "Microbial glycans: the chemistry behind and the biology beyond")
(
link) (
issue)
47.
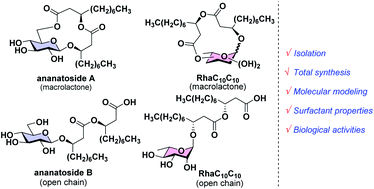
Cloutier, M.; Prévost, M.-J.; Lavoie, S.; Feroldi, T.; Piochon, M.;
Groleau, M.-C.; Legault, J.; Villaume, S.; Crouzet, J.; Dorey, S.; De
Rienzo, M. A. D.; Déziel, E.;
Gauthier, C.* Total
synthesis, isolation, surfactant properties, and biological evaluation
of ananatosides and related macrodilactone-containing rhamnolipids.
Chem. Sci. 2021,
12, 7533-7546 (
link) (
SI)
(
preprint)
46. Muru, K.;
Gauthier, C.* Glycosylation and protecting
group strategies towards the synthesis of saponins and bacterial
oligosaccharides: a personal account.
Chem. Rec. 2021,
11, 2990-3004 (invited contribution to the special issue "Recent advances in carbohydrate chemistry") (
link)
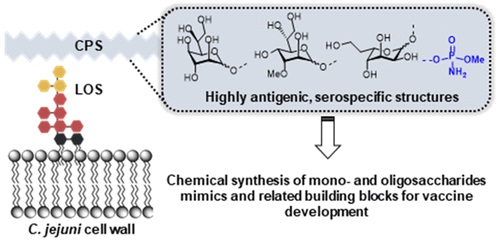
45. Cloutier, M.;
Gauthier, C.* Progress towards the development of glycan-based vaccines against campylobacteriosis.
ACS Infect. Dis. 2021,
7, 969-986 (invited contribution to the special issue "Gut pathogens") (
link) (
issue)
44.
Tremblay, T.; Carpentier, A.; Giguère, D.;
Gauthier, C. Click approach to lipoic acid glycoconjugates. In:
Carbohydrate Chemistry: Proven Synthetic Methods, Volume 5, Ed.: Kosma, P.; Wrodnigg, T. M.; Stütz, A.;
2020, CRC Press, 344 pp
(invited contribution) (
link)
43.
Klaus, J. R.; Majerczyk, C.; Moon, S.; Eppler, N. A.; Smith, S.; Tuma, E.; Groleau, M.-C.; Asfahl, K. L.; Smalle
y, N. E.; Hayden, H.; Piochon, M.; Ball, P. N.; Dandekar, A. A.;
Gauthier, C.; Déziel, E.; Chandler, J. R.
Burkholderia thailandensis methylated hydroxy-alkylquinolines: biosynthesis and antimicrobial activity in co-cultures.
Appl. Environ. Microbiol. 2020,
86, e01452-20 (
preprint) (
link)
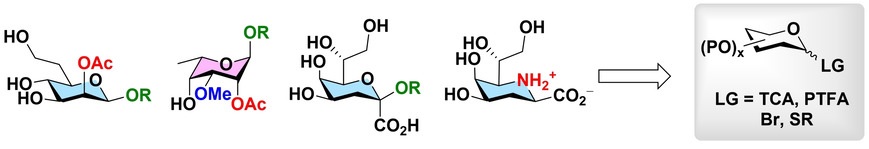
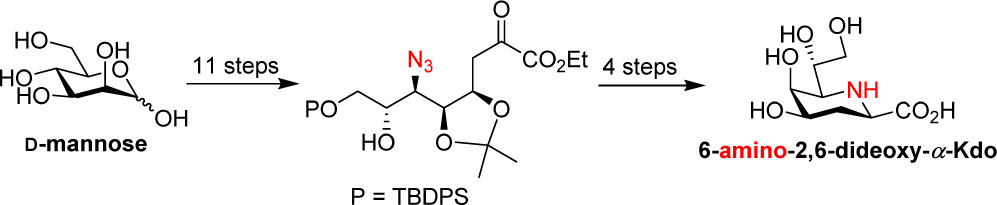
42.
Gamboa Marin, O. J.;
Hussain, N.; Ravicoularamin, G.; Ameur, N.; Gormand, P.; Sauvageau, J.;
Gauthier, C.* Total synthesis of 6-amino-2,6-dideoxy-
α-Kdo from
D-mannose.
Org. Lett. 2020,
22, 5783-5788 (
link) (
SI)
41.
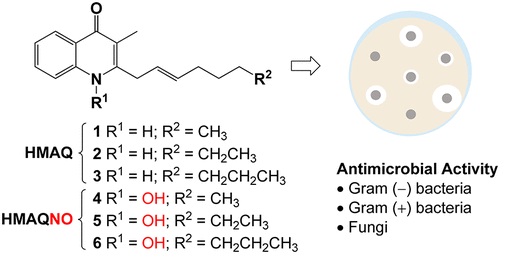
Piochon, M.; Coulon, P. M. L.; Caulet, A.; Groleau, M.-C.; Déziel, E.;
Gauthier, C.* Synthesis and antimicrobial activity of
Burkholderia-related 4-hydroxy-3-methyl-2-alkenylquinolines (HMAQs) and their
N-oxide counterparts.
J. Nat. Prod. 2020,
83, 2145-2154 (
link) (
SI) (
preprint)
40.
Islam, S. T.; Alvarez, I. V.; Saïdi, F.; Giuseppi, A.; Vinogradov, E.;
Morrone, C.; Brasseur, G.; Sharma, G.; Benarouche, A.; Bridot, J.-L.;
Ravicoularamin, G.; Cagna, A.;
Gauthier, C.;
Singer, M.; Fierobe, H.-P.; Mignot, T.; Mauriello, E. M. F. Modulation
of bacterial multicellularity via spatio-specific polysaccharide
secretion.
PLoS Biol. 2020,
18, e3000728 (
link)
39.
Cloutier, M.;
Gauthier, C.* 3-Deoxy-
D-
manno-oct-2-ulosonic acid (Kdo) derivatives in antibacterial drug discovery. In:
Carbohydrates in Drug Discovery and Development, 1st edition, Ed.: Tiwari, V. K.;
2020, Elsevier, 700 pp (invited contribution) (
link)
38.
Cloutier, M.; Muru, K.;
Gauthier, C.* Synthesis of oligosaccharides related to potential bioterrorist pathogens. In:
Recent Trends in Carbohydrate Chemistry,
1st edition, Ed.: Rauter, A. P.; Christensen, B.; Somsak, L.; Kosma,
P.; Adamo, R.;
2020, Elsevier, 532 pp (invited contribution)
(
link)
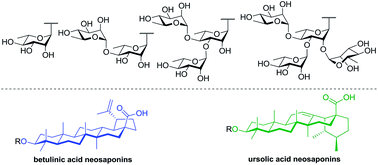
37. Sylla, B.; Lavoie, S.; Legault, J.;
Gauthier, C.*;
Pichette, A. Synthesis, cytotoxicity and anti-inflammatory activity of
rhamnose-containing ursolic and betulinic acid saponins.
RSC Adv. 2019, 9, 39743-39757 (
link) (
SI)
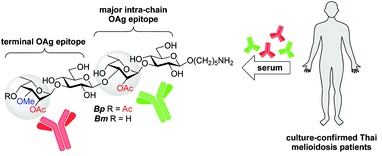
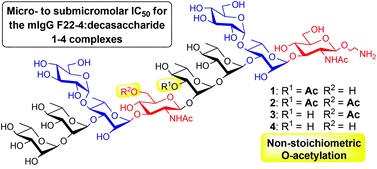
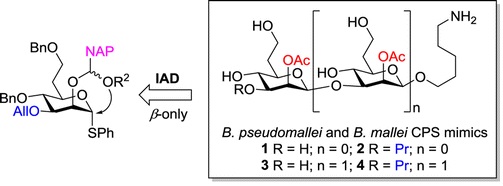
36. Cloutier, M.; Delar, E.; Muru, K.; Ndong, S.; Hoyeck, R. R.;
Kaewarpai, T.; Chantratita, N.; Burtnick, M. N.; Brett, P. J.;
Gauthier, C.* Melioidosis patient serum-reactive synthetic
tetrasaccharides bearing the predominant epitopes of
Burkholderia pseudomallei and
Burkholderia mallei O-antigens.
Org. Biomol. Chem. 2019,
17, 8878-8901 (invited contribution to
the special issue "Glycosylation: New methodologies and applications") (
link) (
SI) (
issue)
35.
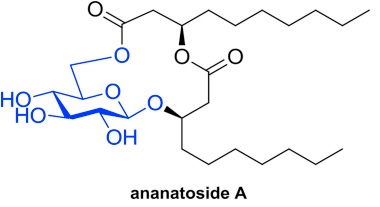
Gauthier, C.*; Lavoie, S.; Piochon M.; Martinez, S.; Milot, S.; Déziel,
E. Structural determination of ananatoside A: an unprecedented
15-membered macrodilactone-containing glycolipid from
Pantoea ananatis.
Carbohydr. Res. 2019,
471, 13-18 (
link) (
SI)
34.
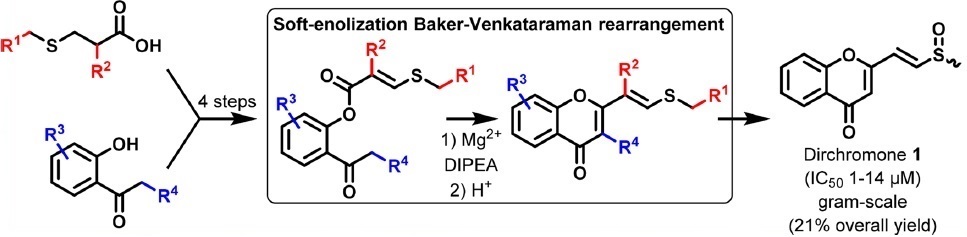
St-Gelais, A.; Alsarraf, J.; Legault, J.;
Gauthier, C.; Pichette, A.
Soft-enolization Baker-Venkataraman rearrangement enabled total
synthesis of dirchromones and related 2-substituted chromones.
Org. Lett. 2018,
20, 7424-7428 (
link) (
SI)
33.
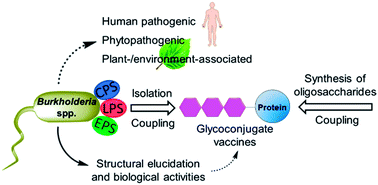
Cloutier, M.; Muru, K.; Ravicoularamin, G.;
Gauthier, C.* Polysaccharides from
Burkholderia species as targets for vaccine development, immunomodulation and chemical synthesis.
Nat. Prod. Rep. 2018,
35, 1251-1293 (
link) (
cover art)
32.
Mihoub, M.; Pichette, A.; Sylla, B.;
Gauthier, C.; Legault, J. Bidesmosidic betulin saponin bearing
L-rhamnopyranoside moieties induces apoptosis and inhibition of lung cancer cells growth
in vitro and
in vivo.
PLoS ONE 2018,
13, e0193386 (
open access)
31.
Tamigney
Kenfack, M.; Mazur, M.; Nualnoi, T.; Shaffer, T. L.;
Ngassimou, A.; Blériot, Y.; Marrot, J.; Marchetti, R.; Sintiprungrat,
K.; Chantratita, N.; Silipo, A.;
Molinaro, A.; AuCoin, D. P.; Burtnick, M. N.; Brett, P. J.;
Gauthier,
C.* Deciphering minimal antigenic epitopes associated with
Burkholderia pseudomallei and
Burkholderia mallei lipopolysaccharide O-antigens.
Nat. Commun. 2017,
8, 115 (
open access) (
SI) (
peer review file) (
merged file)
30.
Lavoie,
S.;
Côté,
I.; Pichette, A.;
Gauthier,
C.; Ouellet, M.; Nagau-Lavoie, F.; Mshvildadze, V.;
Legault, J. Chemical composition
and anti-herpes simplex virus type 1 (HSV-1) activity of extracts from
Cornus canadensis.
BMC Complem. Altern. Med. 2017,
17, 123 (
open access) (
peer review file)
29.
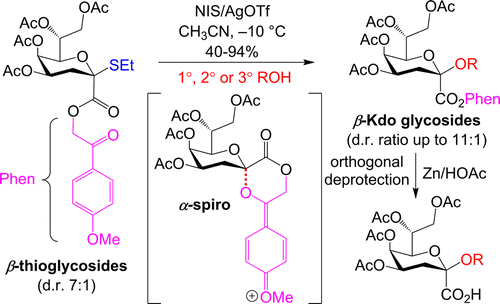
Mazur, M.; Barycza, B.;
Andriamboavonjy, H.; Lavoie, S.; Tamigney Kenfack, M.; Laroussarie, A.;
Blériot, Y.; Gauthier,
C.* 4'-Methoxyphenacyl-assisted
synthesis of β-Kdo glycosides. J. Org. Chem. 2016,
81, 10585-10599 ("Featured Article" & Cover Page
of Issue #22) (
link)
(
SI) (
cover art)
28. Simard, F.;
Gauthier, C.;
Legault, J.; Lavoie, S.; Mshvildadze, V.; Pichette, A. Structure
elucidation of anti-methicillin
resistant
Staphylococcus
aureus (MRSA) flavonoids from balsam poplar buds.
Bioorg. Med. Chem.
2016,
24,
4188-4198
(
link) (
SI)
27.
Lavoie, S.; Ouellet, M.; Fleury,
P.-Y.;
Gauthier, C.;
Legault, J.; Pichette A. Complete
1H and
13C NMR assignments of a series of
pergalloylated tannins.
Magn.
Reson. Chem. 2016,
54, 168-174
(
link) (
SI)
26.
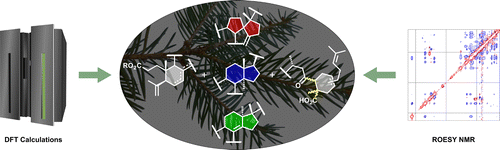
Lavoie
,
S.
†;
Gauthier, C.†;
Mshvildadze, V.; Legault, J.; Roger, B.; Pichette, A. DFT
calculations and ROESY NMR data for the diastereochemical
characterization
of cytotoxic tetraterpenoids from the oleoresin of
Abies balsamea.
J. Nat. Prod. 2015,
78, 2896-2907
(
†Equal contribution) (
link) (
SI)
25.
Laroussarie, A.; Barycza, B.;
Andriamboavonjy, H.; Tamigney Kenfack, M.; Blériot, Y.; Gauthier, C.* Synthesis of the
tetrasaccharide repeating unit of the
β-Kdo-containing
exopolysaccharide from Burkholderia
pseudomallei and B.
cepacia complex. J. Org. Chem. 2015, 80, 10386-10396 (
link) (
SI)
24.
Marchetti, R.; Dillon, M. J.; Burtnick, M.
N.; Hubbard, M. A.; Tamigney Kenfack, M.; Blériot, Y.;
Gauthier, C.; Brett,
P. J.; AuCoin, D. P.; Lanzetta, R.; Silipo, A.; Molinaro, A.
Burkholderia pseudomallei
capsular polysaccharide recognition by a monoclonal antibody reveals
key details toward a biodefense vaccine and diagnostics against
melioidosis.
ACS Chem. Biol. 2015,
10, 2295-2302 (
link) (
SI)
23.
Simard, F.;
Gauthier,
C.; Chiasson, E.; Lavoie, S.; Mshvildadze, V.; Legault,
J.; Pichette, A. Antibacterial
balsacones J-M, hydroxycinnamoylated dihydrochalcones from
Populus balsamifera
buds.
J.
Nat. Prod. 2015,
78,
1147-1153 (
link) (
SI)
22. Blériot, Y.; Tran, A. T.; Prencipe, G.; Jagadeesh, Y.; Auberger, N.;
Zhu, S.;
Gauthier, C.;
Zhang, Y.; Désiré, J.; Adachi, I.; Kato, A.; Sollogoub, M.
Synthesis of 1,2-trans 2-acetamido-2-deoxy-homoiminosugars.
Org. Lett. 2014,
16,
5516-5519 (
link) (
SI)
21.
Blériot, Y.;
Auberger, N.; Jagadeesh, Y.;
Gauthier,
C.;
Prencipe, G.; Tran, A. T.; Marrot, J.; Désiré, J.; Yamamoto, A.; Kato,
A.; Sollogoub, M.
Synthesis of 1,2-cis homoiminosugars
derived from GlcNAc and GalNAc exploiting a β-aminoalcohol skeletal
rearrangement. Org. Lett. 2014, 16, 5512-5515 (
link) (
SI)
20. Sylla
, B.
†;
Gauthier, C.†;
Legault, J.; Fleury, P.-Y.; Lavoie, S.; Mshvildadze, V.; Muzashvili, T.
; Kemertelidze, E.;
Pichette, A.
Isolation
of a new disaccharide nucleoside from Helleborus
caucasicus:
structure elucidation and total synthesis of hellecaucaside A and
its β-anomer.
Carbohydr.
Res. 2014,
398, 80-89
(*Equal contribution) (
link) (
SI)
19.
;
Chassagne, P.; Theillet, F.-X.; Guerreiro, C.; Thouron, F.; Nato, F.;
Delepierre, M.; Sansonetti, P. J.; Phalipon, A.; Mulard, L. A.
.
18.
17. Maaliki,
C.;
Massinon, O.; Sagar, R.; Vincent, S. P.; Blériot, Y
, 418-444
(invited contribution to the special issue in the memory of Pr André Lubineau) (
16.
, S.
Legault, J.; Mercier, S.; Mshvildadze, V.; Pichette, A. Lanostane- and
cycloartane-type triterpenoids from
.